Answer:
D. 2.02 x
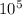 Pa
Pa
Step-by-step explanation:
The gauge pressure is the pressure measured relative to the atmospheric pressure.
Absolute pressure (or total pressure), is the sum of the gauge pressure and the atmospheric pressure. i.e
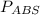 =
=
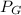 +
+
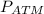
Where;
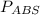 = absolute pressure
= absolute pressure
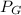 = gauge pressure = 1.01 x
= gauge pressure = 1.01 x
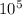 Pa (from the question)
Pa (from the question)
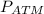 = atmospheric pressure = 1.01 x
= atmospheric pressure = 1.01 x
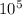 Pa
Pa
Substitute these values into the equation above;
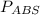 =
=
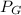 +
+
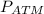
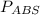 = 1.01 x
= 1.01 x
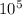 + 1.01 x
+ 1.01 x
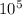
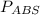 = 2.02 x
= 2.02 x
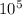 Pa
Pa
Therefore, the absolute pressure of the air inside the balloon is 2.02 x
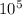 Pa
Pa