Whenever we deal with the problems for checking the amount of heat lost or gained(absorbed) by the fluid, we use the following equation:
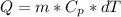
--- (A)
Where,
Q = The amount of heat lost or gained(absorbed) by the fluid
m = Mass of the fluid
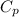
= Heat Capacity of the fluid
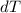
= Change in Temperature = Final Temperature(
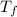
) - Initial Temperature(
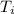
)
Data given:Initial Temperature =
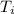
= 23.2°C
The amount of heat absorbed by the water =
347 kJ
Mass of the water = m = 42 kg
Heat Capacity of water =
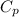 = 4.184 kJ/kg°C
= 4.184 kJ/kg°C
Final Temperature =
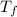 = ?
= ?
Plug-in the values in equation (A)
(A) => 347 = 42 * 4.184 * (
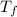 - 23.2)
- 23.2)
=>
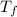 ≈ 25.175°C
≈ 25.175°C
Ans: The final temperature of water ≈ 25.175°C
-i