In case you're in hurry:
Ans: 26 valence electrons
Step-by-step explanation:To find the valence electrons, we need to add the valence electrons of the individual atom in the compound. Therefore, let us divide this question into 3 parts.
1) What is the number of valence electrons of Arsenic (As)?
2) What is the number of valence electrons of Oxygen (O)?
3) What is the total number of valence electrons of
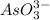
1.
The number of valence electrons of Arsenic(As) = 5
2.
The number of valence electrons of Oxygen (O) = 6
3.
Since there are 3 atoms of Oxygen, and 3 extra electrons are also present in the shape of "3-", as it is an ion, therefore,
The total number of valence electrons of
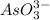
= 5 + 6 * 3 + 3 =
26 valence electrons.
Ans: The total number of valence electrons in 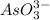 = 26.
= 26.
-i