16 g/ml
Further explanation
Given
mass = 32.40 g
volume = 2.0mL
Required
Density
Solution
Density is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
Density formula:
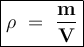
ρ = density
m = mass
v = volume
Input the value :
ρ = 32.40 g / 2.0 ml
ρ = 16.2 g/ml
Rules for division: The least number of significant figures in the problem determines the number of significant figures in the answer.
32.40 = 4 sig.fig
2.0 = 2 sig fig
The answer must be 2 sig fig
So the answer = 16 g/ml