The new volume : 21.85 ml
Further explanation
Given
V1=25,0 ml
P1=725 mmHg
T1=298K is converted to
T2=273'K
P2=760 mmHg atm
Required
V2
Solution
Combined gas law :
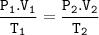
Input the value :
V2=(P1.V1.T2)/(P2.T1)
V2=(725 x 25 ml x 273)/(760 x 298)
V2=21.85 ml