Answer: Potential energy of products is less than the potential energy of the reactants.
Step-by-step explanation:
Exothermic reactions are defined as the reactions which releases heat. The release in heat is due to the difference in the potential energy of the reactants and the products.
For these reactions, the potential energy of the products is less than the potential energy of the reactants. Total enthalpy change of the reaction is given by the equation:
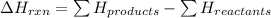
For exothermic reaction,
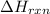 is negative.
is negative.
For the reaction of baking soda and vinegar, the equation follows:
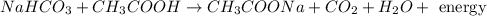
As, the energy is written at the product side, this means that the reaction between baking soda an=d vinegar is an exothermic reaction.