Answer: Option (a) is the correct answer.
Step-by-step explanation:
Molar mass is defined as the mass in grams of one mole of a substance.
Mathematically, Molar mass =
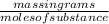
For example, molar mass of 1 mole of
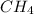 molecule will be calculated as follows.
molecule will be calculated as follows.
Molar mass of
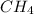 =
=
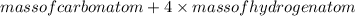
=
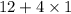
= 16 g
Therefore, 1 mole of
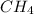 molecules has a molar mass of 16 g.
molecules has a molar mass of 16 g.