Answer: Option (C) is the correct answer.
Step-by-step explanation:
A compound that is formed by transfer of electrons results in the formation of an ionic bond and it is known as an ionic compound.
For example, calcium has 2 valence electrons and bromine has 7 valence electrons. So, in order to complete their octet calcium donates its two valence electrons to two bromine atoms.
Therefore, it results in the formation of an ionic compound
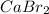 .
.
In an ionic compound there is partial positive charge on the cation and partial negative charge on the anion.
Whereas a compound formed by sharing of electrons is known as a covalent compound.
For example,
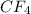 ,
,
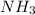 and
and
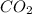 are all covalent compounds.
are all covalent compounds.
Thus, we can conclude that
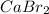 is the compound that is held together by the electrostatic force between two ions.
is the compound that is held together by the electrostatic force between two ions.