The Molarity of the solution is given by:
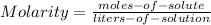
--- (A)
But NaCl, which is a solute, is given in grams.
Therefore, first, we need to convert grams into moles.
Molecular weight of NaCl = 58.44 grams/mole.
So,
117 grams * (moles/ 58.44 grams) =
2.00 moles of NaClNow that we have moles of NaCl, plug in the values in (A):
(A) => Molarity = (2 / 2.7 ) =
0.741 moles/litreThe correct answer is:
0.741 moles/litre = Molarity