Answer: B) Iron rusts in water.
Step-by-step explanation:
Physical property is defined as the property of a substance which becomes evident during physical changes. Example: Melting point , electrical conductivity, malleability,
Chemical property is defined as the property of a substance which becomes evident during chemical changes. Example: Reactivity with other substances
Rust is hydrated ferric oxide
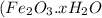 .
.
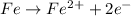
Corrosion of iron is called as rusting. Rust is hydrated ferric oxide
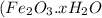 .
.
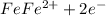
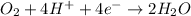
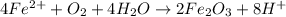
Thus rusting of iron is a chemical property.