Answer:
73.2g
Step-by-step explanation:
The reaction expression is given as:
P₄ + 6Cl₂ → 4PCl₃
Given parameters:
Volume of chlorine gas = 79.2L
Unknown:
Mass of Phosphorus needed = ?
Solution:
To solve this problem, let us find the number of moles of the chlorine gas.
Since the condition of the reaction is at STP;
22.4L of gas is contained in 1 mole
79.2L of chlorine gas will contain
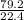 = 3.54mole
= 3.54mole
From the reaction expression;
6 moles of chlorine gas will react with 1 mole of P₄
3.54 mole of chlorine gas will completely react with
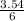 = 0.59mole of P₄
= 0.59mole of P₄
Mass of P₄ = number of moles x molar mass
Molar mass of P₄ = 4 x 31 = 124g/mol
Mass of P₄ = 0.59 x 124 = 73.2g