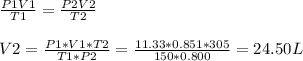Answer:
Original pressure = P1 = 11.3 atm
Final volume = V2 = 24.5 L
Step-by-step explanation:
Given data:
Initial conditions:
Volume V1 = 0.851 L
Temperature T1 = 150.0 K
Moles of He n1 = 0.783
From ideal gas equation:
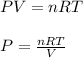
Substituting the initial V, T and n using R = 0.0821 L.atm/mol-K we get:
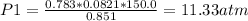
Final conditions:
T2 = 305 K
P2 = 0.800 atm
Since the number of moles of He will remain constant we can write: