The piece of iron is brought from a temperature of 100C to a temperature of 22C. In this process, the heat released by this piece of iron is
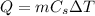
where
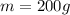
is the mass of the piece of iron,
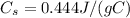
is the iron's specific heat, and the temperature variation is
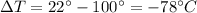
. Using these values, the amount of heat released by the iron is
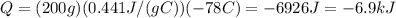
With negative sign because it is amount of heat released. This heat is then trasferred to the water, so the amount of heat absorbed by the water is Q=6.9 kJ.