To solve this we are going to use the half life equation
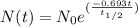
Where:
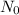
is the initial sample
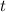
is the time in years
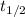
is the half life of the substance
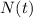
is the remainder quantity after
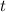
years
From the problem we know that:
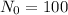
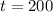
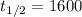
Lets replace those values in our equation to find
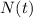
:
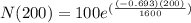
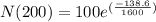
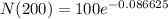
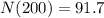
We can conclude that after 1600 years of radioactive decay, the mass of the 100-gram sample will be
91.7 grams.