First, we need to find the atomic mass of
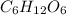
.
According to the periodic table:
The atomic mass of Carbon = C = 12.01
The atomic mass of Hydrogen = H = 1.008
The atomic mass of Oxygen = O = 16
As there are 6 Carbons, 12 Hydrogens and 6 Oxygens, therefore:
The
molar mass of
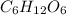
= 6 * 12.01 + 12 * 1.008 + 6 * 16
The
molar mass of
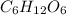
= 180.156
grams/moleNow that we have the molar mass of
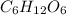
, we can find the grams of glucose by using:
mass(of glucose in grams) = moles(of glucose given in moles) * molar mass(in grams/mole)
Therefore,
mass(of glucose in grams) = 2.47 * 180.156
mass(of glucose in grams = 444.99 grams
Ans: Mass of glucose in grams in 2.47 moles =
444.99 grams
-i