Answer:
![Rate=k[A]^1[B]^5[C]^6](https://img.qammunity.org/2019/formulas/chemistry/college/szp3jcu32spp60dfg1ulutu0xjjqx5irfk.png)
Step-by-step explanation: Rate law says that rate of a reaction is directly proportional to the concentration of the reactants each raised to a stoichiometric coefficient determined experimentally called as order.
Order of the reaction is defined as the sum of the concentration of terms on which the rate of the reaction actually depends. It is the sum of the exponents of the molar concentration in the rate law expression.
Elementary reactions are defined as the reactions for which the order of the reaction is same as its molecularity and order with respect to each reactant is equal to its stoichiometric coefficient as represented in the balanced chemical reaction.
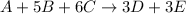
![Rate=k[A]^1[B]^5[C]^6](https://img.qammunity.org/2019/formulas/chemistry/college/szp3jcu32spp60dfg1ulutu0xjjqx5irfk.png)
k= rate constant
1 = order with respect to A
5 = order with respect to B
6 = order with respect to C
Thus rate law is
![Rate=k[A]^1[B]^5[C]^6](https://img.qammunity.org/2019/formulas/chemistry/college/szp3jcu32spp60dfg1ulutu0xjjqx5irfk.png)