Answer:
The percentage of potassium in the given complex is 35.54 %.
Step-by-step explanation:
Mass of potassium in
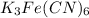 = 3 × 39.10 g mol=117.3 g/mol
= 3 × 39.10 g mol=117.3 g/mol
Molar mass of
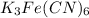 =329.15 g/mol
=329.15 g/mol
Percentage of potassium (K) in the the complex:
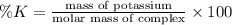
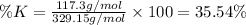
The percentage of potassium in the given complex is 35.54 %.