Answer: The correct answer is Option d.
Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios. It is expressed as
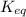
For a general chemical reaction:
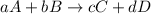
The expression for
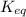 is written as:
is written as:
![K_(eq)=([C]^c[D]^d)/([A]^a[B]^b)](https://img.qammunity.org/2019/formulas/chemistry/college/8i6z9kdl5sbqqw6gt2ligbic9v48km7t6n.png)
There are 3 conditions:
- When
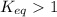 ; the reaction is product favored.
; the reaction is product favored. - When
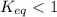 ; the reaction is reactant favored.
; the reaction is reactant favored. - When
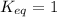 ; the reaction is in equilibrium.
; the reaction is in equilibrium.
From the above expression, the equilibrium constant is directly dependent on product concentration. Thus, more is the concentration of product, more will be the equilibrium constant.
The highest values of
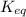 will favor the product more.
will favor the product more.
Hence, the correct answer is Option d.