Answer:
F and Cu.
Step-by-step explanation:
Hello,
In this case, by knowing that the farther the elements in the periodic table, the more ionic they are, in addition to the difference in the electronegativity which must be greater than 1.7 for the bond to be ionic, we can substantiate that the iron and the copper don't exhibit an ionic bond as shown below:
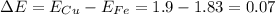
Such value is characteristic for covalent bonds.
Best regards.