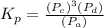Answer:
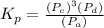
Step-by-step explanation:
The equilibrium constant is expressed as the relationship between the molar concentration of reagents and products. The expression of a generic reaction is:
aA + bB <--------> cC + dD
![K_(p)=([C]^(c) [D]^(d) )/([A]^(a) [B]^(b))](https://img.qammunity.org/2019/formulas/chemistry/high-school/jkijztuvbq82fam0jg28k67bs01qc0afn3.png)
The numerator is the product of the concentrations of the products and the denominator is the product of the reagents. Each term in the equation is raised to a power whose value is that of the stoichiometric coefficient in the balanced equation.
When it comes to gas mixtures, it is sometimes more appropriate to describe the composition in terms of partial pressures. So in this case we will have:
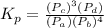
As the concentration and partial pressure of pure liquids and solids can be considered as 1, the final equation will be: