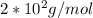Answer:
The molar mass of the unknown gas is 200 g/mol. R:
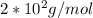
Step-by-step explanation:
We have an effusion experiment with oxygen and another unknown substance.
Oxygen effuses (v1) through a tiny hole 2.5 times faster than unknown substances (v2). It means
v1 = 2.5v2.
Molar mas of Oxygen is M1 = 32.0 g/mol.
This process can be studied by using Graham law.
![(v1)/(v2) = \sqrt[2]{(M2)/(M1) }](https://img.qammunity.org/2019/formulas/chemistry/college/506cugu0n0t4sl72jgpyauj4jn51ls7l5j.png)
Where M2 is the unknown molecular mass, all the other data are given in the problem. Replacing and isolating M2. we can fin its value:
![(v1)/(v2) = \sqrt[2]{(M2)/(M1) } \\v1 = 2.5 v2\\(2.5v2)/(v2) = \sqrt[2]{(M2)/(M1) } \\\\2.5 = \sqrt[2]{(M2)/(M1) } \\(M2)/(M1) = (2.5)^2\\M2 = (2.5)^2 M1 = (2.5)^2*32.0 (g)/(mol)\\ M2 = 6.25* 32.0 (g)/(mol) = 200 (g)/(mol)\\M2 = 200 (g)/(mol)](https://img.qammunity.org/2019/formulas/chemistry/college/1jmsebqyvsn8ccdt56agtuhnq2rftw6m08.png)
The molar mass of the unknown gas is 200 g/mol. R: