Answer:
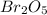
Step-by-step explanation:
Hello,
Considering the given information, the undergoing chemical reaction is:

Thus, we consider the formed grams of
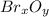 because the grams of bromine are equal before and after the chemical reaction (mass can't be neither created nor destroyed), thus, the bromine grams into the
because the grams of bromine are equal before and after the chemical reaction (mass can't be neither created nor destroyed), thus, the bromine grams into the
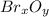 are 1.250g and the oxygen grams that come from the ozone are:
are 1.250g and the oxygen grams that come from the ozone are:

Now, we compute the moles for each one of them as:
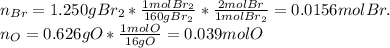
Now, we divide by the bromine's moles to find the littlest whole-number that allows us to identify the empirical formula as shown below:
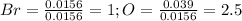
Finally, by multiplying by two to find the littlest whole-number, one gets:
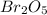
Best regards.