Answer: 110 cal
It is given that he specific heat of Iron is
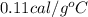 .
.
Mass of iron fork 20 g
Heat energy is needed to raise the temperature of this from
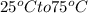
we can use the following formula to calculate heat energy:
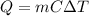
where Q is the heat required to raise the temperature by
 of a mass m having specific heat C.
of a mass m having specific heat C.
Insert the values:
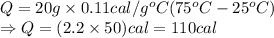
Thus, heat required to raise the temperature of iron fork is 110 cal.