Step-by-step explanation:
No. of moles of cholesterol solute,
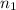 = 0.99 mol
= 0.99 mol
No. of moles of toulene solvent,
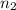 = 5.4 mol
= 5.4 mol
Hence, total number of moles of solution will be as follows.
n =
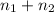
= 0.99 mol + 5.4 mol
= 6.39 mol
Therefore, mole fraction of cholestrol solute (
 ) is as follows.
) is as follows.
=
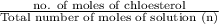
 =
=
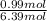
= 0.154
Vapor pressure of pure toulene solvent (
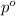 ) = 41 torr
) = 41 torr
Vapor pressure of solution = P
Hence, formula to calculate relative lowering of vapor pressure is as follows.
Relative lowering of vapor pressure =

As per relative lowering of vapor pressure colligative property, the relative lowering of vapor pressure is equal to the mole fraction of solute.
Hence,
 =
=

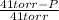 = 0.154
= 0.154
41 torr - P = 6.314
P = 34.686 torr
or, = 35 torr
Therefore, we can conclude that vapor pressure of the solution is 35 torr.