Answer:
Container will rupture at temperature of 375 K.
Step-by-step explanation:
Initial pressure of the nitrogen gas =
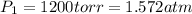
(1 torr = 0.00131 atm)
Initial temperature of nitrogen gas =
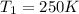
Final pressure of the nitrogen gas =
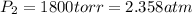
Final temperature of nitrogen gas =
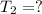
Since, the container is inflexible that is volume remains constant we can apply Gay Lussac's law:
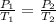
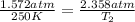
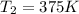
Container will rupture at temperature of 375 K.