Answer:
Backward direction
Step-by-step explanation:
Exothermic reaction: It is that reaction in which heat is evolved during reaction.
We are given
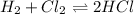
It is exothermic reaction.
We know that in exothermic reaction when we increase the temperature then the equilibrium shift in backward direction and when we decrease the temperature then the equilibrium shift in forward direction.
Therefore, when we increasing the temperature then the equilibrium shift in backward direction.