Answer:
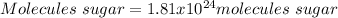
Step-by-step explanation:
Hello,
In this case, Avogadro's number help us to realize that 1 mole of any substance has 6.022x10²³ molecules of the same substance, in such way, by applying that relationship one computed the consumed molecules of sugar as shown below:
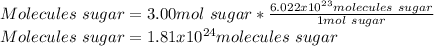
Best regards.