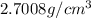Answer : The density of a piece of metal is
Explanation :
To calculate the volume of cube, we use the formula:
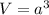
where,
a = edge length of cube
Given :
Edge length of cube = 2.50 cm
Volume of cube =
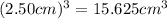
Given :
Mass of metal = 42.20 g
To calculate density of a substance, we use the equation:
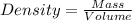
Putting values in above equation, we get:
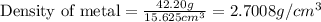
Hence, the density of a piece of metal is