To calculate moles , we divide mass of the substance given by the molar mass of the substance.
That can be mathematically represented as:
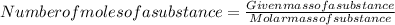
Here given mass of substance is 4540 g
Molar mass of the substance is 196 g mol⁻¹
So number of moles can be calculated as:
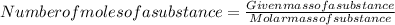
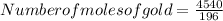
So number of moles of gold =23.16 mol