Hello!
The concentration of the final solution when a
chemistry teacher adds 50.0 mL of 1.50 M H2SO4 solution to 200 mL of water is 0,3 M
To calculate that, you'll need to use the dilution law, where initial and final concentrations are M1 and M2 respectively, and initial and final volumes are V1 and V2, as shown below. Keep in mind that the final volume is the sum of the 200 mL of water and the 50 mL of H₂SO₄ that were added by the teacher.
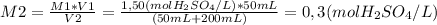
Have a nice day!