This problem tests the concept of general gas equation, which is:
PV = nRTWhere,
P = Pressure
V = Volume
n = number of moles
R = General gas constant
T = Temprature
We are to find V, and we have the rest of the values.
P = 183.24 KPa = 183240 Pa
n = 0.215 moles
T = 75 K
R = 8.314 J /(mol.K)
Using the values in the equation, we get:
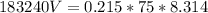
V = 0.000732 m³
The value of volume is cubic meter. In cubic cm, the value of volume will be:
V = 0.000732 x 1000000 = 732 cm³