Answer:
631.3 milligrams of oxygen gas will react with the TNT.
Step-by-step explanation:
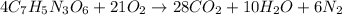
Moles of TNT =
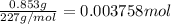
According to reaction , 4 moles of tNT react with 21 moles of oxygen gas.
Then 0.003758 moles of TNT will react with:
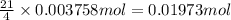
Mass of 0.01973 moles of oxygen ;
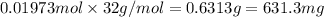
( 1 g = 1000 mg)
631.3 milligrams of oxygen gas will react with the TNT.