Answer:
3.3 atm
Step-by-step explanation:
This is a simple application of Dalton's law of partial pressure which state that the total pressure exerted by a mixture of gas is the sum of the individual partial pressure of the component gases.
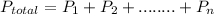
Hence, the total pressure of the gas mixture becomes:
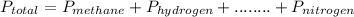
= 1 + 1.2 + 1.1
= 3.3 atm
The total pressure of the mixture is 3.3 atm