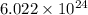Answer: The number of ammonium ions in given moles of ammonium sulfide are
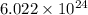
Step-by-step explanation:
We are given:
A chemical compound having chemical formula of
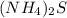
In 1 mole of ammonium sulfide, 2 moles of ammonium ions and 1 mole of sulfide ions are present.
According to mole concept:
1 mole of compound contains
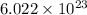 number of atoms
number of atoms
So, 5 moles of ammonium sulfide molecule will contain =
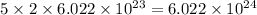 number of ammonium ions.
number of ammonium ions.
Hence, the number of ammonium ions in given moles of ammonium sulfide are