Answer:
The percentage is 50.7%
Step-by-step explanation:
The formula of chromium (III) carbonate is (Cr₂(CO₃)₃)
Let us calculate the molar mass of (Cr₂(CO₃)₃)
molar mass of (Cr₂(CO₃)₃) =
2X atomic mass of Cr + 3X atomic mass of C + 9X atomic mass of O
molar mass of (Cr₂(CO₃)₃) = 2X 52 + 3X12 +9X16 = 284g/mol
As per the formula there are nine moles of oxygen in each mole of (Cr₂(CO₃)₃).
so we can say that
In 284g of (Cr₂(CO₃)₃) the mass of oxygen = 144g
so 1 g of (Cr₂(CO₃)₃) the mass of oxygen =
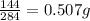
Therefore
the mass of oxygen in 100g of(Cr₂(CO₃)₃) =0.507X100=50.7
Thus percentage of Oxygen in (Cr₂(CO₃)₃) =50.7%