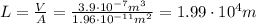(a) First of all, let's convert the gold's density into appropriate units:
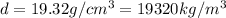
and the mass as well:
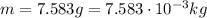
From density and mass, we can find the volume of the leaf, V:
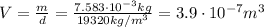
We know that the thickness is
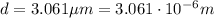
, and the volume is the product between the thickness and the area:
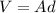
, so we can find the area:
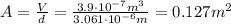
(b) The radius of the cylinder is
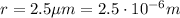
, therefore its area is
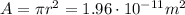
For a cylinder, the volume is the product between the length L and the area A: V=AL, therefore we can find the length L (the volume is the one calculated at the previous step):