The first law of thermodynamics says that the variation of internal energy
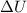
of a gas is equal to the amount of heat Q supplied to the gas minus the work W done by the gas:
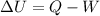
The variation of internal energy of a gas is:
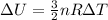
As it can be seen, it depends only on the variation of temperature

. Since for an isothermal process
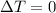
, then
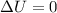
. This means that the first law of thermodynamics becomes
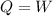
and since the work done is 370 J, then the amount of heat is also 370 J:
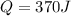
.