Answer:
Aluminium bromide
Step-by-step explanation:
Thinking process:
The aluminium bromide has the following structure:
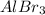
The compound is a Lewis acid.
Aluminium bromide has a vacant p-orbital so it accepts a lone pair of electrons and acts as a Lewis acid.
Sodium bromide, on the other hand, donates electrons and acts as a Lewis base like this:
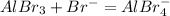
Thus, clearly the bromide ion is a base, and it donates electrons.