The electron charge is equal to
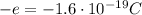
. The atomic nucleus of the problem has a charge of
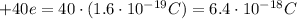
. The distance between the nucleus and the electron is
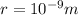
, so we can calculate the electrostatic (Coulomb) force between the two:
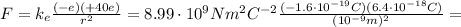
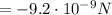
which is attractive, since the two charges have opposite sign.