Answer: Option (c) is the correct answer.
Step-by-step explanation:
A molecule which has hydrogen bonding will have the highest boiling point. So, out of the given options only
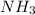 will have hydrogen bonding.
will have hydrogen bonding.
Whereas in
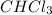 there will be dipole-dipole interactions and no hydrogen bonding within the molecule.
there will be dipole-dipole interactions and no hydrogen bonding within the molecule.
In
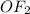 and
and
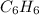 , there will be dipole-dipole interaction in both the molecules.
, there will be dipole-dipole interaction in both the molecules.
Thus, we can conclude that
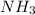 will have the highest boiling point.
will have the highest boiling point.