First all, the decay formula is
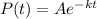
where:
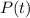
is the remaining quantity after
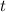
years
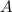
is the initial sample
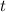
is the time in years
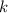
is the decay constant
From the problem we know that
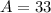
and
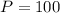
, but we don't have the time
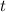
; to find it we will take advantage of the half-life of the Carbon-14. If you have a sample of 100 mg and Carbon-14 has a half-life of 5730, after 5730 years you will have half of your original sample i.e. 50 mg. We also know that after
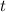
years we have a remaining sample of 33mg, so the amount of the sample that decayed is
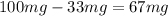
. Knowing all of this we can set up a rule 3 and solve it to find
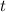
:
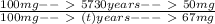
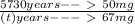
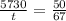
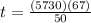
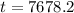
Now that we know our time
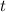
lets replace all the values into our decay formula:
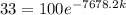
Notice that the constant
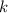
we need to find is the exponent; we must use logarithms to bring it down, but first lets isolate the exponential expression:
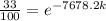
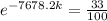
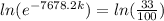
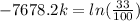
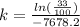

We can conclude that the decay constant
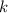
is approximately -0.000144