Boyle's law of ideal gas: This law states that the volume of a gas is inversely proportional to its pressure at a constant temperature. Acc to this law we can write the relation of pressure and volume as:
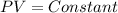
That means:
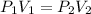
From that equation we can calculate Volume of gas at a certain pressure:
P₁=Initial pressure
V₁=Initial volume
P₂=Final pressure
V₂= Final volume
Here P₁, initial pressure is given as 85.0 kPa
V₁, initial volume is given as 525 mL
P₂, final pressure is 65.0 kPa
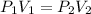
so,
V_{2}=85\times 525\div 65
=686 mL
Volume of gas will be 686 mL.