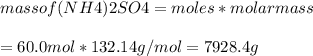Ans: D) 7930 g
Given:
Moles of H2SO4 = 60.0 mol
NH3 in excess
To determine:
The amount in grams of (NH4)2SO4 produced
Step-by-step explanation:
The chemical reaction is as follows:
2NH3 + H2SO4 → (NH4)2SO4
Since, NH3 is in excess, H2SO4 will be the limiting reagent and will influence the amount of product formed
Based on the reaction stoichiometry:
1 mole of H2SO4 forms 1 mole of (NH4)2SO4
Therefore, 60.0 mol of H2SO4 will produce 60.0 mol of (NH4)2SO4
Now:
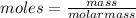
molar mass (NH4)2SO4 = 132.14 g/mol