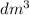Firstly, according to Ideal Gas Law the volume of 1 mole of a gas at Standard Temperature and Pressure (STP) is 22.4
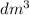 , ie the value of the molar volume (Vm) is 22.4
, ie the value of the molar volume (Vm) is 22.4
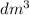 /mol.
/mol.
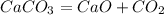
From the reaction, it can be seen that
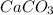 and
and
 have the following amount of substance relationship:
have the following amount of substance relationship:
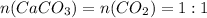
From the relationship we can determinate moles of
 :
:
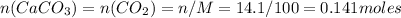
Finally, we can calculate the volume of formed CO2:
V(
 )=nxVm=0.141x22.4=3.16
)=nxVm=0.141x22.4=3.16