Answer : The energy evolved in the reaction is 1499.52 kJ
Explanation : Given,
Mass of Fe = 197 g
Molar mass of Fe = 56 g/mole
Enthalpy of reaction = -852 kJ
First we have to calculate the moles of Fe.
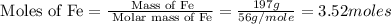
Now we have to calculate the energy evolved in the reaction.
From the balanced chemical reaction we conclude that,
As, 2 mole of Fe evolved energy = 852 kJ
So, 3.52 mole of Fe evolved energy =
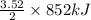
= 1499.52 kJ
Therefore, the energy evolved in the reaction is 1499.52 kJ