Answer:
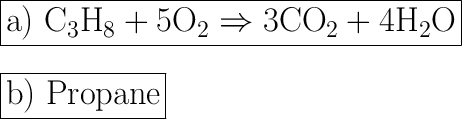
![\rule[225]{225}{2}](https://img.qammunity.org/2019/formulas/chemistry/middle-school/f96ewg0co75mc2m1c03wn0rirt8yxybabr.png)
Step-by-step explanation:
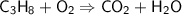
Balancing Carbon atoms on the right side,
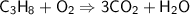
Balancing Hydrogen atoms on the right side,
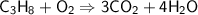
Balancing Oxygen atoms on the left side,
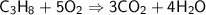
The limiting reactant is Propane, since Oxygen will not be running out. The amount of Propane is limited.
![\rule[225]{225}{2}](https://img.qammunity.org/2019/formulas/chemistry/middle-school/f96ewg0co75mc2m1c03wn0rirt8yxybabr.png)