The formula of specific gravity is:
Specific gravity =
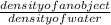
To get this, you can derive a formula for mass. If you transpose the density of water to the other side of the equation (by specific gravity) you will multiply it with specific gravity. Now the density of water is 1 g/mL so you will retain the value of the specific gravity. That will just leave you with this formula:
Specific gravity = density of an objectDensity of an object can be computed using the formula:
Density = mass/volume now you can fuse the two formulas to get this:
Specific gravity = mass/volume
From there we can derive the formula for mass by transposing volume to the other side of the equation:
Volume x specific gravity = mass
Lets's use your given in our new equation:
10.0 mL x 1.04 = mass
10.4 = mass
The mass of a 10.0 mL sample of urine with a specific gravity of 1.04 is
10.4g.