Answer:
The amount of substance we need to recover 34.6 mol of carbon is 465.47g
Step-by-step explanation:
we want to know what mass of substance we need to recover 34.6 mol of carbon
This substance is composed of 89.2% carbon
Wmust recover 34.6 mol of carbon and we know that the molecular mass of carbon is 12g / mol this means that one mole of carbon has a mass of 12 g
We use a simple rule of three to know how many grams of carbon are in 34.6 mol
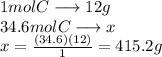
415.2 g are 34.6 mol of carbon
As the amount of carbon we want to recover is 415.2g (34.6mol) and this amount corresponds to 89.2% of the total
To calculate the total mass we need we use a simple rule of three
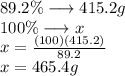
The amount of substance we need to recover 34.6 mol of carbon is 465.47g