the correct answer is "they cause the same number of electrons to be ejected".
In fact, in the photoelectric effect, the number of photoelectrons emitted does not depend on the frequency of the light, but only on its intensity. The frequency of the light affects only the kinetic energy of the photoelectrons, because each photon carries an energy of
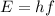
where h is the Plank constant and f is the frequency of the wave, and then this energy is converted into kinetic energy of the photoelectron. But the number of photoelectrons emitted depends only on the number of incoming photons, so it depends only on the intensity of the light. Therefore, violet and blue light cause the emission of the same number of photoelectrons.