Answer: 228.8 gStep-by-step explanation:1) Chemical equation, balanced: given
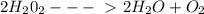
2) mole ratios
2 mol H2O2 : 2 mol H2O :1 mol O2
3) proportion with the unknown quantity of O2:
2 mol H2O2 14.3 mol H2O2
------------------- = -----------------------
1mol O2 x
4) Solve for x:
x = 14.3 mol H2O2 * 1 mol O2 / 2 mol H2O2 = 7.15 mol O2
5) Convert 7.15 mol O2 to grams
molar mass = mass in grams / number of moles
=> mass in grams = number of moles * molar mass
molar mass of O2 = 2 * 16.00 g/mol = 32.00 g/mol
mass in grams O2 = 7.15 moles * 32.00 g / mol = 228.8 g
Answer: 228.8 g